Bonding in Coordination Compounds
Bonding in Coordination Compounds: Overview
This Topic covers sub-topics such as Bonding in Coordination Compounds
Important Questions on Bonding in Coordination Compounds
Which of the following compound has hybridization, square planar shape, and diamagnetic behaviour?
Ammonia forms the complex ion with copper ions in alkaline solution but not in acidic solution. The probable reason for this is:
Nickel combines with a uninegative monodentate ligand to form a paramagnetic complex . The number of unpaired electron/s in nickel and geometry of this complex ion are respectively _____.
A square planar complex is formed by hybridisation of which atomic orbitals?
If is replaced by in the complex , which of the following properties are expected to get changed?
A. Geometry
B. Geometrical isomerism
C. Optical isomerism
D. Magnetic properties
The correct order of the number of unpaired electrons in the given complexes is
(A)
(B)
(C)
(D)
(E)
Choose the correct answer from the options given below:
Which of the following complex is octahedral, diamagnetic and the most stable?
Match List-I with List-II
| LIST-I Coordination Complex |
LIST-II Number of unpaired electrons |
||
| A. | I. | 0 | |
| B. | II. | 3 | |
| C. | III. | 2 | |
| D. | IV. | 4 |
Choose the correct answer from the options given below:
Which of the following is most stable, diamagnetic and octahedral in shape?
In 20th century, German scientist Werner succeeded in clarifying the structures of the five compounds consisting of platinum, chlorine, and ammonia. Some of the properties of these compounds are shown below in the table.
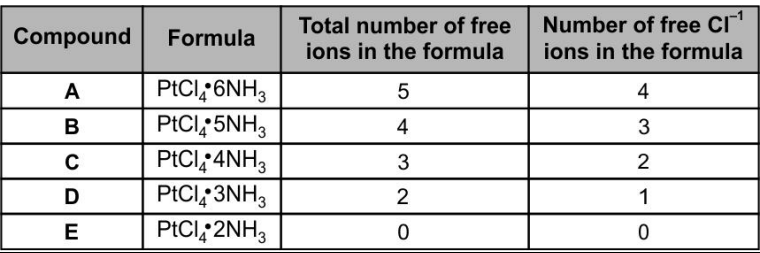
Predict the shape of each compound.
Determine the hybridisation, geometry and magnetism of the following complex.
Explain the hybridisation of hexammine chromium (III) ion.
Find the maximum number of atoms lying in the one plane for .
The shapes of the following coordination complexes are
The structure of which of the following chloro species can be explained on the basis of hybridisation?
In , the hybridisation of central atom and number of unpaired electron can be respectively
How you can give a geometrical shape for a complex entities by its formula only?
Which of the following compound has tetrahedral geometry?
Which of the following is an outer orbital complex?
The hybridisation of in is
